Gastroenterological Surgery | New Targets Identified for Treating Liver Metastases in Colorectal Cancer
结直肠癌肝转移靶向糖基化修饰治疗找到新靶点,胃肠外科团队新发现
2025-01-17
Recently, a pioneering study, titled N-glycosylation modification of CTSD affects liver metastases in colorectal cancer, by Professor Shen Zhanlong and Professor Ye Yingjiang from the Department of Gastroenterological Surgery at Peking University People's Hospital (PKUPH), in collaboration with Professor Li Yan from the Institute of Biophysics of the Chinese Academy of Sciences (IBP), was published in the prestigious journal Advanced Science. Dr. Xiong Nan and Dr. Du Yan from the same department at PKUPH and Associate Researcher Huang Chuncui from IBP are the co-first authors of the paper, while Professors Shen Zhanlong, Ye Yingjiang, and Li Yan are the co-corresponding authors.

Liver metastasis is the primary factor
contributing to unfavorable prognosis in colorectal cancer (CRC), which ranks
among the top 5 deadliest cancers globally. Approximately 80–90% of patients
with liver metastases cannot access effective treatment. There is an urgent
need to explore new mechanisms and develop innovative strategies to address
this clinical challenge.
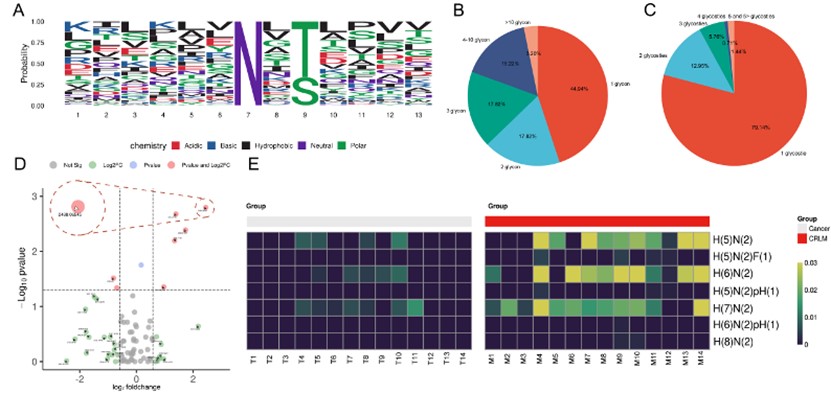
However, there are only scattered and single studies in this field. It is necessary to conduct comprehensive studies addressing the N-glycosylation proteomics associated with liver metastasis in CRC. Through mass spectrometry, the team conducted a comprehensive comparison. They analyzed the N-glycosylation modification protein expression profiles in primary lesions and paired liver metastatic lesions of CRC by Liquid Chromatography-Mass Spectroscopy (LC-MS) analysis. It was found that N-glycosylation modification at residue 263 of Cathepsin D (CTSD) affects liver metastasis in CRC. This modification affects the cellular localization, stability, and enzymatic activity of CTSD. Furthermore, it influences the invasion and metastasis of CRC cells by modulating the ferroptosis pathway mediated by ACADM. These findings may provide potential therapeutic targets for CRC treatment as well as strategies for controlling CRC progression and metastasis.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/advs.202411740?af=R

