Dermatology | Prof. Zhang Jianzhong’s Team Publishes Phase III Study on New Atopic Dermatitis Drug Stapokibart in Renowned Journal
皮科牵头的特应皮炎创新药司普奇拜单抗III期临床研究在权威杂志发表
2024-11-19
On October 28, 2024, the team led by
Professor Zhang Jianzhong from the Department of Dermatology at Peking
University People's Hospital published a study titled Long-term efficacy and
safety of stapokibart for moderate-to-severe atopic dermatitis: 52-week results
from a phase 3 trial, in the prestigious journal Allergy (IF=12.6). Dr.
Zhao Yan from the same department is the first author of this paper and
Professor Zhang Jianzhong is the corresponding author.

Atopic Dermatitis (AD) is a common chronic
inflammatory skin disease that significantly impacts patients' quality of life.
Traditional treatments for moderate-to-severe AD include systemic corticosteroids and immunosuppressants. Although these therapies have
shown efficacy, they are often accompanied by many side effects. The Phase III
trial results from Professor Zhang Jianzhong and his team provided evidence of
a highly effective new therapy with a high safety profile, widely recognized by the international
academic community.

Stapokibart is a humanized monoclonal antibody targeting interleukin-4 receptor α subunit (IL-4Rα), a shared receptor for IL-4 and IL-13 which are key pathogenic drivers of AD. The study observed 500 patients with moderate-to-severe AD, randomly assigned in a 1:1 ratio to receive one of two therapies: stapokibart and placebo, for 16 weeks. After the 16-week double-blind treatment, patients in both stapokibart and placebo groups entered a 36-week maintenance treatment period during which they received stapokibart 300 mg every 2 weeks. The concomitant use of topical medications for AD was permitted throughout the maintenance period. Of the 476 patients who entered the maintenance period, 430 subjects completed the treatment. At week 52, EASI-75 (Eczema Area and Severity Index) was achieved in 92.5% of patients continuing stapokibart and 88.7% of those switching from placebo to stapokibart, respectively; an IGA (Investigator's Global Assessment) score of 0/1 with a ≥2-point reduction was achieved in 67.3% and 64.2% of patients, respectively; a ≥4-point reduction in weekly average of daily Peak Pruritus Numerical Rating Scale (PP-NRS) was achieved in 67.3% and 60.5% of patients, respectively.
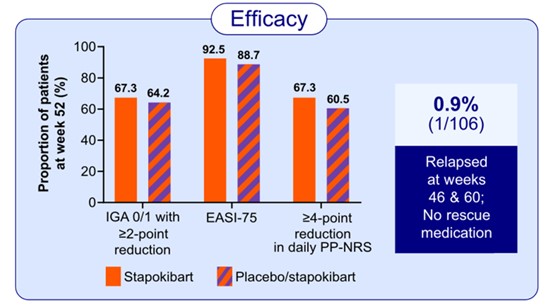
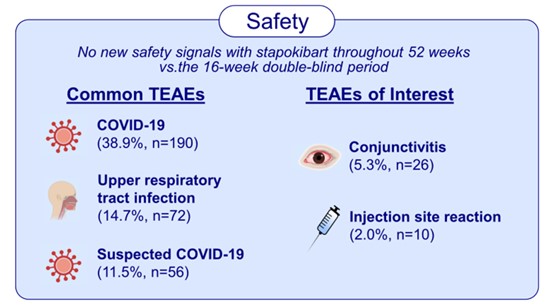
The study has shown sustained efficacy and a favorable safety profile for long-term treatment with stapokibart in adult patients with moderate-to-severe AD. These findings highlight stapokibart as a promising long-term therapy for patients with this chronic condition. This study provides a foundation for the clinical use of stapokibart and inspires the advancement of targeted therapies for AD in China.
Paper Link: https://pubmed.ncbi.nlm.nih.gov/39450683/

