Rheumatology and Immunology | New Research Findings by Prof. Li Zhanguo and Prof. Hu Fanlei’s Team Published in Annals of the Rheumatic Diseases
栗占国、胡凡磊团队关于肠道病毒通过分子模拟机制诱发类风湿关节炎研究成果在权威期刊发表
2024-09-29
The team led by Professor Li Zhanguo and Professor Hu Fanlei from the Department of Rheumatology and Immunology at Peking University People’s Hospital(PKUPH)published a research paper, titled "Rheumatoid arthritis patients harbor aberrant enteric bacteriophages with autoimmunity-provoking potential: a paired sibling study", in the prestigious journal Annals of the Rheumatic Diseases (IF=20.3).

Professor Hu Fanlei, Dr. Li Xin from PKUPH and Dr. Liu Kai from the Shanghai Institute of Immunity and Infection of Chinese Academy of Sciences are co-first authors of the paper, while Professors Li Zhanguo and Hu Fanlei serve as co-corresponding authors. Important contributions to this research were made by Researcher Zhang Chiyu and Associate Researcher Li Yanpeng from the Shanghai Public Health Clinical Center (SPHCC), as well as Researcher Wang Jun from the Institute of Microbiology, Chinese Academy of Sciences. This study received strong support from various official science foundation programs.
Microbes, especially viruses, have been thought to be important participants in the development of rheumatoid arthritis (RA). However, the profile of enteric virome and its role in RA remains elusive. This study aimed to investigate the atlas and involvement of virome in RA pathogenesis.
The team collected fecal samples from 30 pairs of RA and healthy siblings to minimize genetic interferences for metagenomic sequencing. They analyzed the α and β diversity of the virome and the virome-bacteriome interaction, identified the differential bacteriophages and analyzed their correlations with clinical and immunological features of RA. Furthermore, through auxiliary metabolic gene annotation and molecular mimicry study, they investigated the potential involvement of these differential bacteriophages in RA pathogenesis. The responses of CD4+ T cells and B cells to the mimotopes derived from the differential bacteriophages were also systemically studied.
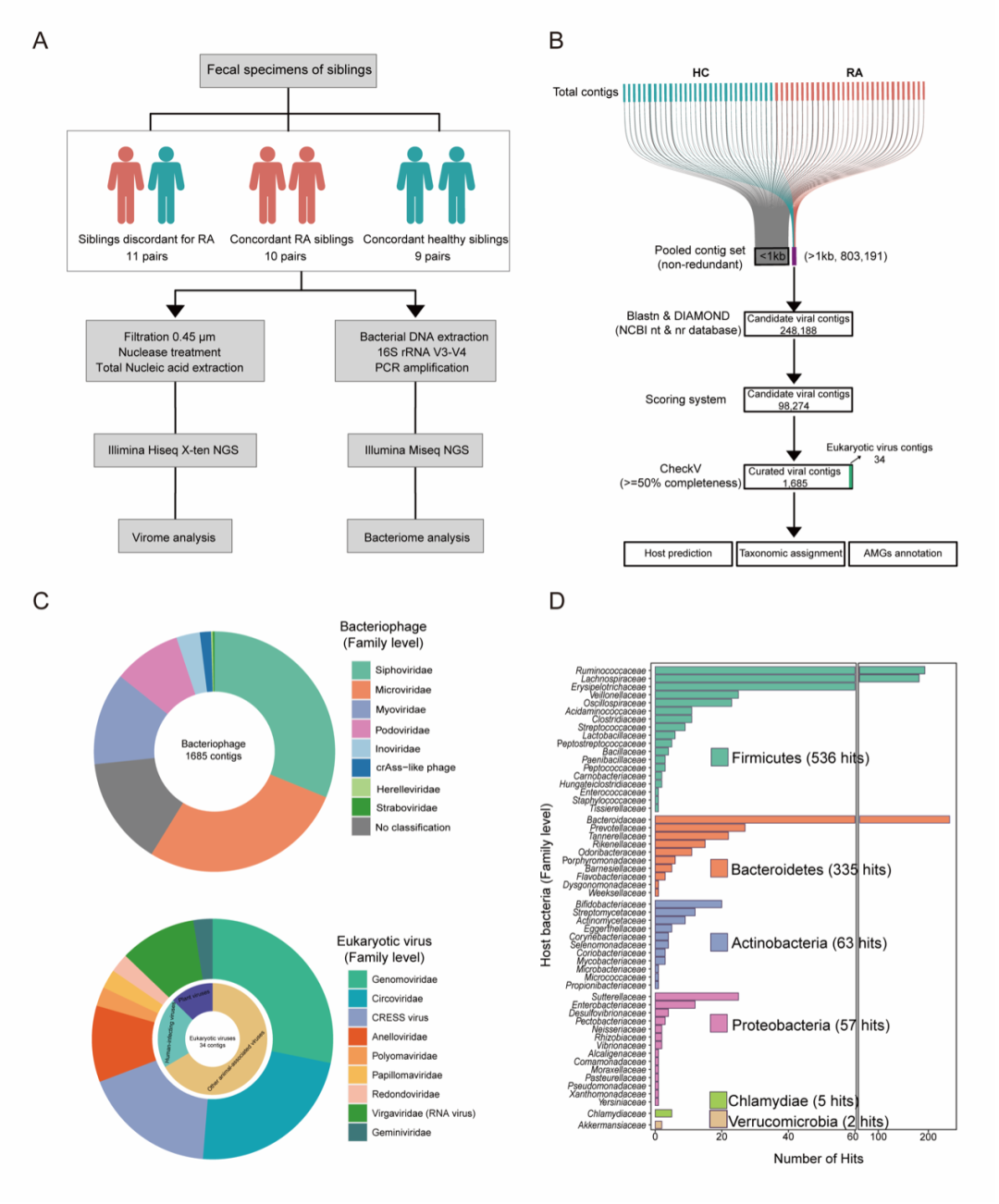
The research exposed that the composition of the enteric bacteriophageome was distorted in RA. The differentially presented bacteriophages correlated with the immunological features of RA, including the anti-CCP autoantibody and HLA-DR shared epitope. Intriguingly, the glycerolipid and purine metabolic genes were highly active in the bacteriophages from RA. Moreover, peptides of RA-enriched phages, in particular Prevotella phage and Oscillibacter phage could provoke the autoimmune responses in CD4+ T cells and plasma cells via molecular mimicry of the disease-associated autoantigen epitopes, especially those of BiP (immunoglobulin binding protein).
This study provides new insightsenteric bacteriophageome in RA development. In particular, the aberrant bacteriophages demonstrated autoimmunity-provoking potential that would promote the occurrence of the disease. Based on these new findings, the targeted therapy against these aberrant enteric bacteriophages may inspire new RA treatment strategies.
Paper Link: http://pubmed.ncbi.nlm.nih.gov/39084885/

