Rheumatology and Immunology | Collaborative Research on Rheumatoid Arthritis by Teams led by Prof. Li Zhanguo and Prof. Hu Fanlei from PKUPH and Prof. Liu Wanli from IITU Published in a Prestigious Journal
栗占国、胡凡磊团队关于类风湿关节炎B细胞免疫调控新机制成果在权威杂志发表
2024-03-04
On February 1, 2024, in a significant
breakthrough in the field of rheumatology, teams led by Professor Li Zhanguo
and Professor Hu Fanlei from the Department of Rheumatology & Immunology,
Peking University People's Hospital (PKUPH), in collaboration with Professor
Liu Wanli from the Institute for Immunology at Tsinghua University (IITU),
published a research paper titled "Proinflammatory Phenotype of B10 and
B10pro cells elicited by TNF-α in Rheumatoid Arthritis" in the top-ranked
journal in the field of rheumatology, Annals of the Rheumatic Diseases (IF:
27.4).
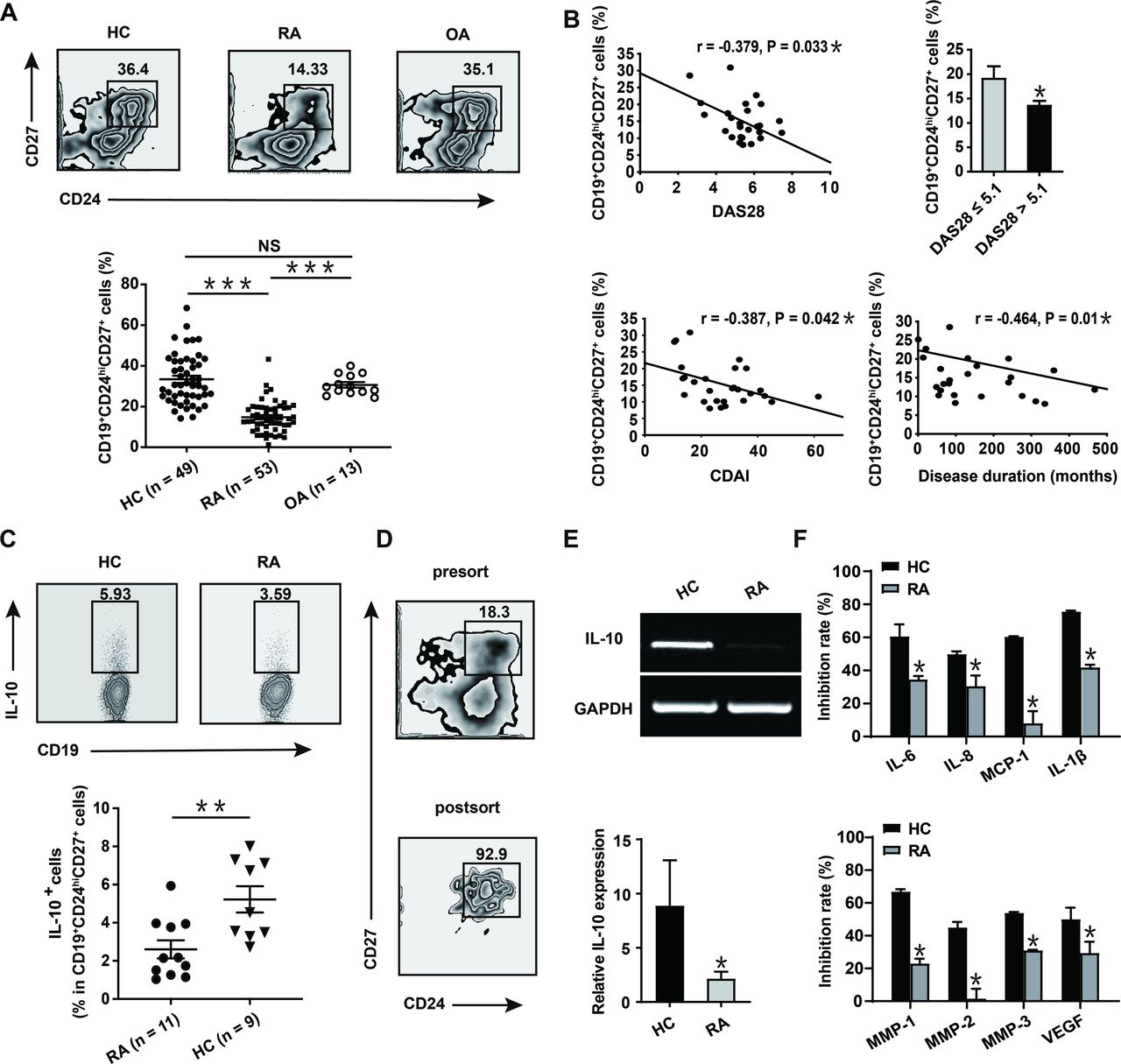
In this study, it has been found that in
rheumatoid arthritis (RA) patients, B10 cells were decreased with
proinflammatory conversion. Moreover, the frequencies of these proinflammatory
B10 cells showed a significant positive correlation with the clinical
manifestations of RA patients. TNF-α, which is significantly elevated in RA,
was found to be the core regulator of B10 cells. It dampened the stability and
differentiation of B10 cells by inhibiting the key regulator SHIP-1. Biologics
targeting TNF-α could restore the immunosuppressive phenotype of B10 cells,
thus exerting therapeutic effects against RA. This provided a new immunological
mechanism for the great success of TNF-α-targeted biologics in treating
autoimmune diseases.
Rheumatoid arthritis (RA) is a highly
disabling autoimmune disease characterized by chronic destructive joint
lesions, affecting multiple organs of the body. The pathogenesis of RA remains
incompletely understood. While TNF-α-targeted biologics have shown significant
therapeutic effects in RA, some patients revealed drug resistance. Therefore,
the pathogenesis of RA and the therapeutic mechanisms of TNF-α-targeted
biologics are of particular interest to the scientists of clinical immunology
and basic immunology.
The abnormal activation of autoreactive B
cells recognizing self-antigens is strongly associated with the development and
progression of RA. The autoantibodies produced by these B cells serve as
diagnostic biomarkers for RA. B cell-targeted depletion demonstrated
significant RA therapeutic effects. Regulatory B10 cells are a newly identified
subset of B cells, exerting negative regulatory functions and maintaining
immune tolerance. Nevertheless, the key regulators and molecular mechanisms
governing B10 cells remain elusive.
In the collaborative research led by PKUPH
and IITU, through follow-up study of RA patients, clinical specimen testing,
and a series of immunological, cellular, molecular, and bioinformatics studies,
the research team revealed the decrease of B10 cells in RA patients, with the
remaining B10 cells exhibiting proinflammatory conversion. TNF-α, which is
significantly elevated in RA, was found to be the core regulator of the number
and function of B10 cells. Moreover, anti-TNF-α therapy could increase the frequencies
of B10 cells in RA patients and restore their immunosuppressive phenotype of
IL-10 secretion, thereby alleviating the symptoms of RA.
These results elucidated a new
immunological mechanism for anti-TNF-α therapy in RA. This mechanism also
warrants further investigationother autoimmune diseases that could be
treated with anti-TNF-α.
According to the research findings, the
application of autologous amplified B10 cells for the clinical treatment of
autoimmune diseases such as RA should be performed with great caution, as these
cells might promote the disease progression due to the proinflammatory
phenotype under an autoimmune milieu.
Paper Link:
https://ard.bmj.com/content/early/2024/02/01/ard-2023-224878

