Rheumatology and Immunology | The Lancet Publishes Research on New Progress in Clinical Treatment of Rheumatoid Arthritis by Prof. Li Zhanguo’s Team
2021-04-18
In March 2021, the Lancet Regional Health published online the results of a large sample multicenter study on the treatment of rheumatoid arthritis titled "Effectiveness and Safety of Rheumatoid Arthritis in Patients with Active RA in Chinese: A Nationwide, Prospective Real World Study" by Prof. Li Zhanguo’s team of Peking University People's Hospital.
This prospective large sample real world study on the treatment of rheumatoid arthritis with national class 1.1 new drug Iguratimod (IGU) provides further evidence for the efficacy and safety of Iguratimod (IGU) in the treatment of rheumatoid arthritis, as well as its clinical application in different types of patients.
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic and aggressive arthritis. Patients without active treatment can eventually lead to joint deformity and functional loss. Disease-modifying anti-rheumatic drugs (DMARDs) are the core of RA treatment.
However, due to the heterogeneity of the response of RA patients to treatment, the currently available DMARDs cannot meet the clinical needs. Despite advances in the treatment of rheumatoid arthritis (RA), approximately 40% of RA patients still do not achieve primary clinical outcomes in randomized trials. More convenient, safe, effective and cheap new therapeutic drugs are needed in daily clinical practice.
This study was supported by the 11th Five-Year-Plan for Science and Technology Support Program and the Key Special Project of Rheumatoid Arthritis of Beijing Science and Technology Commission. Led by Peking University People's Hospital, this study enrolled 1,759 patients with active rheumatoid arthritis from 48 hospitals in China.
The results showed that:
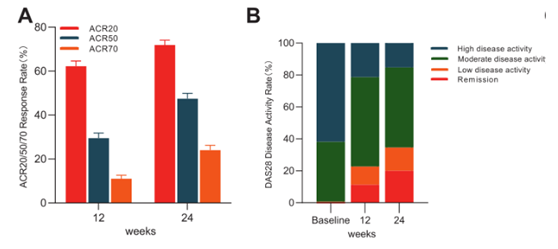
- 71.9% of the patients who received the treatment of Iguratimod (IGU) improved their condition, and the disease activity decreased significantly, especially in the patients with short course of disease.
- In the age stratification analysis, it was found that the efficacy and safety of Iguratimod in patients aged 18-85 years were similar to that of young patients, and there was no significant correlation between age and adverse reactions, suggesting that Iguratimod has better safety in the treatment of elderly RA patients (≤ 85 years old).
- In this study, 0.7% of patients developed SAEs associated with IGU. The most common clinical significant AEs were infection in 0.6% of the patients, abdominal discomfort in 0.5% of the patients including 0.2% gastric ulcer, fracture in 0.4%, and increased alanine aminotransferase (ALT) in 0.2% of the patients.
- In this study, 12 RA patients with pulmonary interstitial lesions had stable pulmonary lesions without aggravation. Only one patient (0.05%) had progress in pulmonary fibrosis during treatment. The results suggested that in RA patients with pulmonary fibrosis, Iguratimod (IGU) may be safe.
- At the same time, compared with previous studies, there were no new adverse reactions in this large sample study, which is one of the main conclusions of this real world study. The analysis of related factors indicated that male patients and patients with short course of disease had better response to treatment.
The Clinical Immunology Center / Department of Rheumatology and Immunology / Rheumatic Immunology Research Institute of Peking University People's Hospital is a national key clinical discipline, innovative team of the Ministry of Education, Beijing Key Laboratory of Rheumatology, Beijing International Science and Technology Cooperation Base, and Asia Pacific Rheumatology Center.
The Department of Rheumatology and Immunology of Peking University People's Hospital has always been committed to the basic and clinical research on rheumatoid arthritis (RA), and has made innovative achievements with theoretical significance and clinical application. The researchers at the Department have found a new RA pathogenic antigen and its pathogenic mechanism, and established a classification standard for early RA (E-RA) and a variety of immunodiagnostic methods. They also have established a series of diagnosis methods for rheumatic disease and clinical immunology, and completed clinical studies such as epidemiological investigation of RA prevalence and disability rate.
This real-world study is another achievement that the team has made through many years of efforts and a lot of preliminary work, which provides further evidence for the better application of Iguratimod in the treatment of RA. It is of great significance for clinical treatment and will benefit more RA patients.
About the author

Prof. Li Zhanguo, chief physician, director of the Clinical Immunology Center, Peking University People's Hospital, director of the Department of Rheumatology and Immunology, Peking University Health Science Center, chief scientist of the National Basic Research Program (973 Program), winner of the National Science Fund for Distinguished Young Scholars, chair of the Clinical Immunology Branch of the Chinese Society of Immunology, former chair of the International League of Associations for Rheumatology (ILAR) and Asia Pacific League of Association for Rheumatology (APLAR), member of WHO Committee on Bone and Muscle Diseases (ICC), chief editor of Chinese Journal of Rheumatology, and associate editoreditorial board member of Clinical Rheumatology, IJRD, Nat Rev Rheum and lancet Rheum. He has been engaged in the research of clinical diagnosis and treatment of rheumatic immune diseases for a long time, and found new pathogenic molecules and mechanisms of rheumatoid arthritis. The established immune diagnosis and treatment methods and schemes have been used in clinical practice. More than 300 SCI papers have been published in NAT Med, Immunity, Science, Lancet Rheum and Cell H & M. More than 20 books including Rheumatoid Arthritis, Advanced Course of Rheumatic Immunology and Kelly Rheumatology were edited / translated by him.

